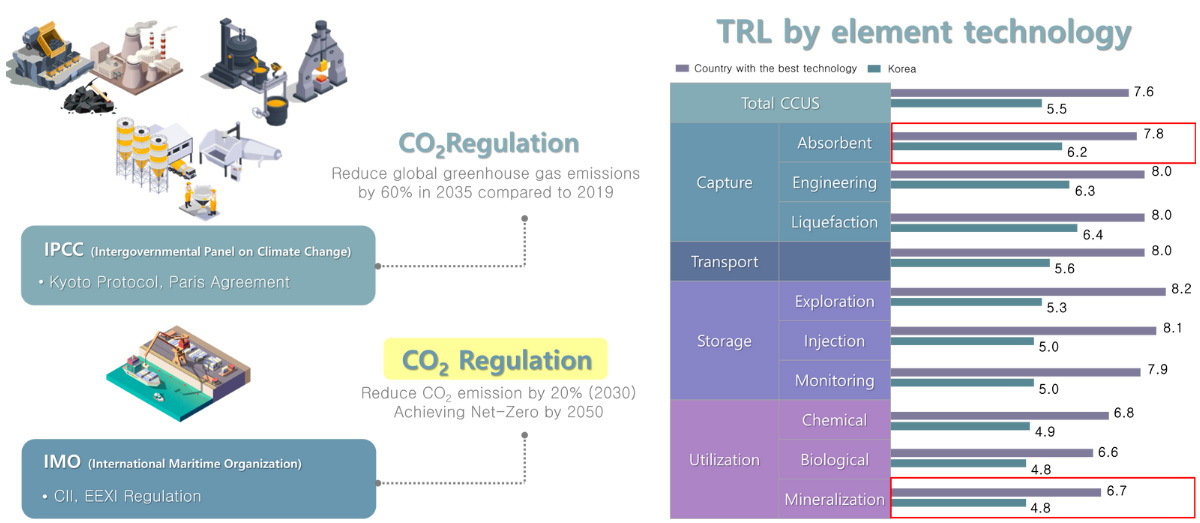
Various CO2 reduction regulations are being created and strengthened through international agreements. In response to this, technological advancements are being made to capture, storage Utilization of CO2
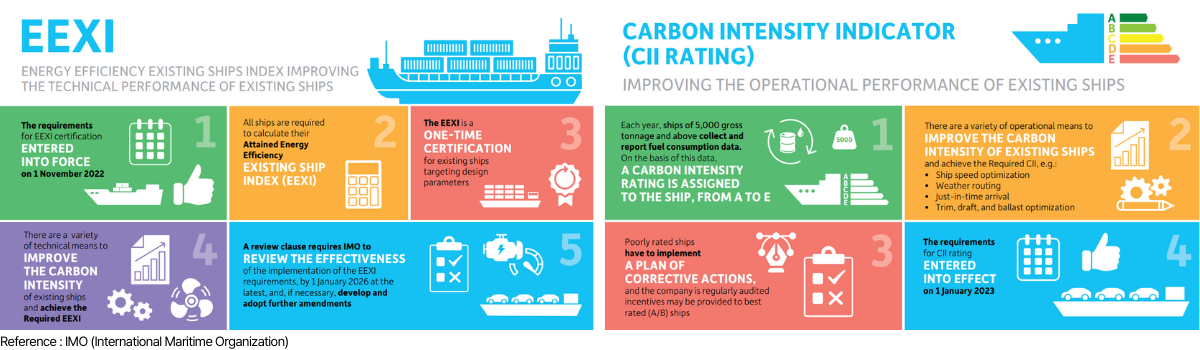
IMO (International Maritime Organization) is creating and strengthening greenhouse gas emission regulations
from ships. As regulations are applied to existing ships, low-speed operation and ship improvement
technologies are being implemented, but there are limits to achieving Net-Zero on marine ships that operate
based on fossil fuels.
IMO regulations in force
[1] EEXI : A preliminary calculation of the amount of CO2 emitted when transporting 1 ton of cargo
for 1 mile
using ship specifications
such as engine power and weight tonnage (applied to ships of 400 tons or less)
[2] CII : The amount of CO2 emitted when transporting 1 ton of cargo for 1 nautical mile is
calculated using
operational information
such as fuel usage and operating distance (applied to ships of 5,000 tons or more)
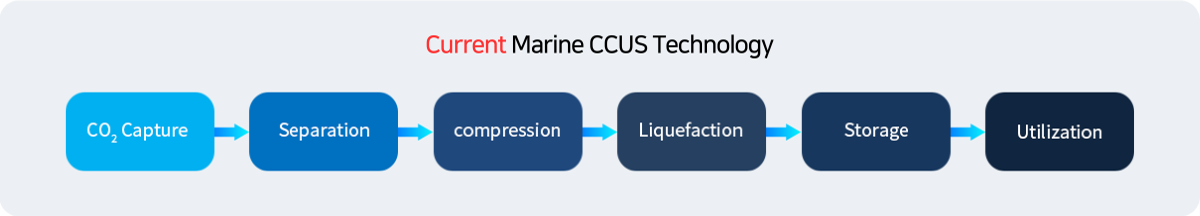
CCU technology is being discussed to solve these problems, but there are problems with the limitation of energy consumption required for CO2 capture and separation on ships, lack of space within limited ships, and storage and utilization of separated CO2 by-products. This technology differentiates itself by dramatically reducing facility operation energy and securing space through optimized design.
CCUS Technology Applied to ships
Response to IMO (International Maritime Organization) greenhouse gas (Green House Gas) emission regulations IMO aims to reduce greenhouse gas emissions from ships to zero by 2050.

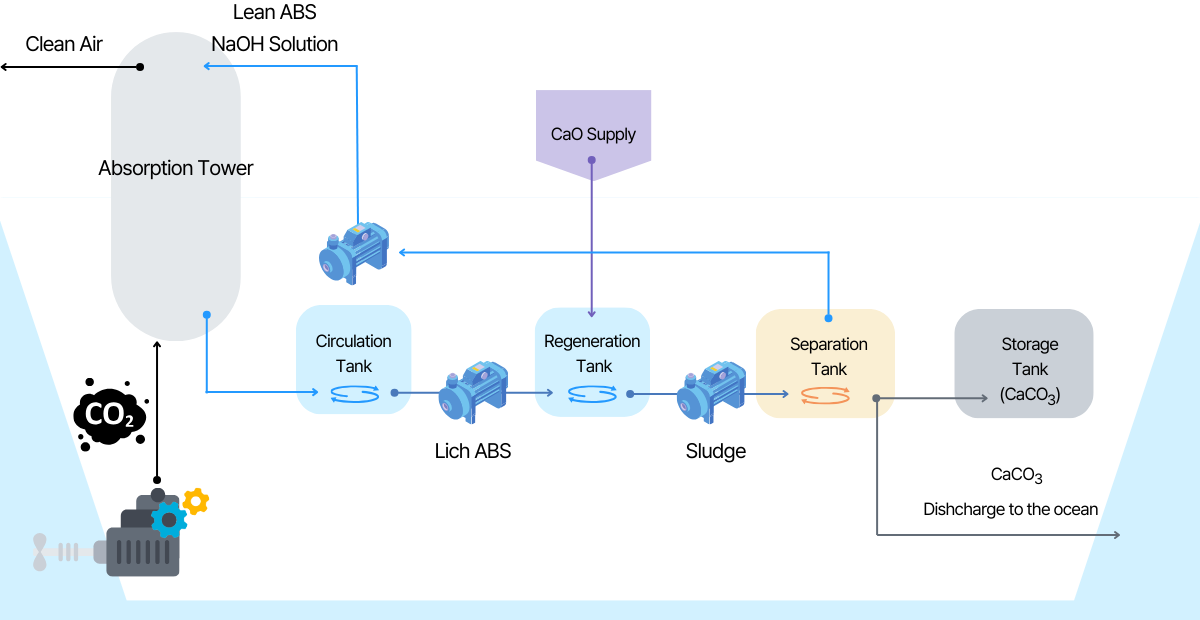
Na2CO3 is created by capturing CO2 using NaOH, a strong alkali. It shows an absorption rate equivalent to or
higher than MEA in the pH range of 14 ~12. Afterwards, CaO is added to the saturated absorption liquid to
produce an insoluble salt, CaCO3, and Na2CO3 is regenerated into NaOH. By regenerating the absorbent using ionic
bonding and solubility differences without a separate pressurization or high-temperature desorption process, it
is possible to dramatically reduce facility operation energy and secure space through optimized design. CaCO3,
which uses low-cost CaO as an intermediate additive and safely stores CO2, can be unloaded on land and used in
industry, or discharged into the sea and isolated according to environmental impact assessment.
[1] CO2 Absorption reaction
2NaOH (aq) + H2CO3 (aq) -> Na2CO3 (aq) + 2H2O (l)
[2] NaOH Regeneration reaction
Na2CO3 (aq) + CaO (s) + H2O (l) -> 2NaOH (aq) + CaCO3 (s) Precipitation
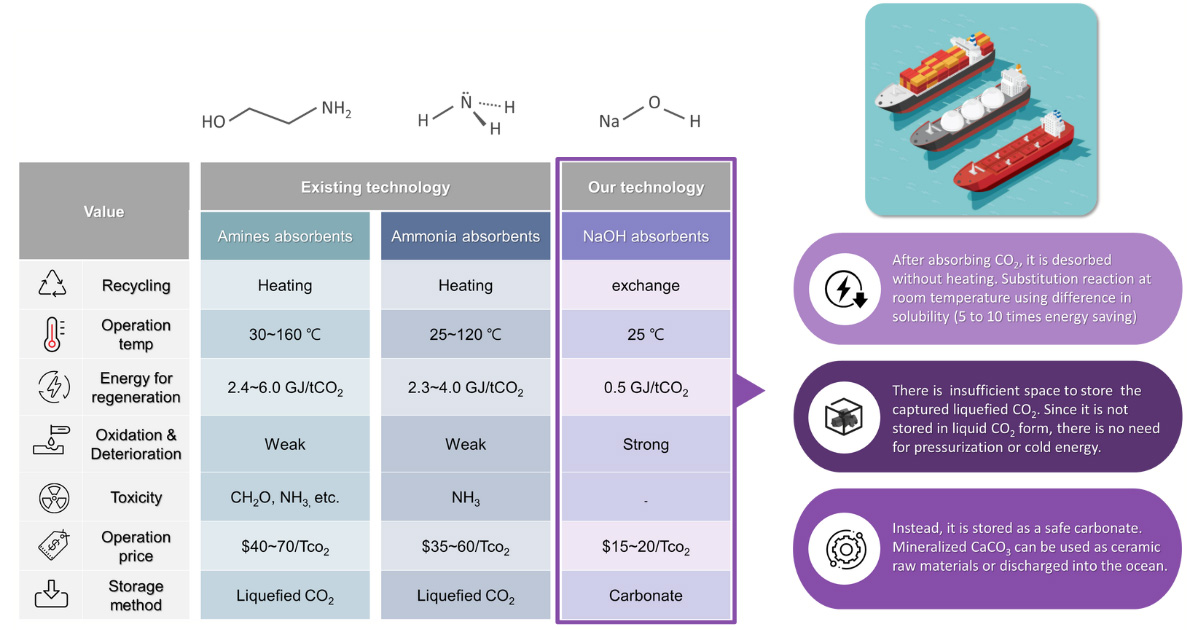
Technology comparison - capture & storage
To capture CO2 on ships, NaOH, an inorganic alkali salt, is used as an absorbent. This complements the shortcomings of organic absorbents such as amines and ammonia applied in existing CCUS technology.
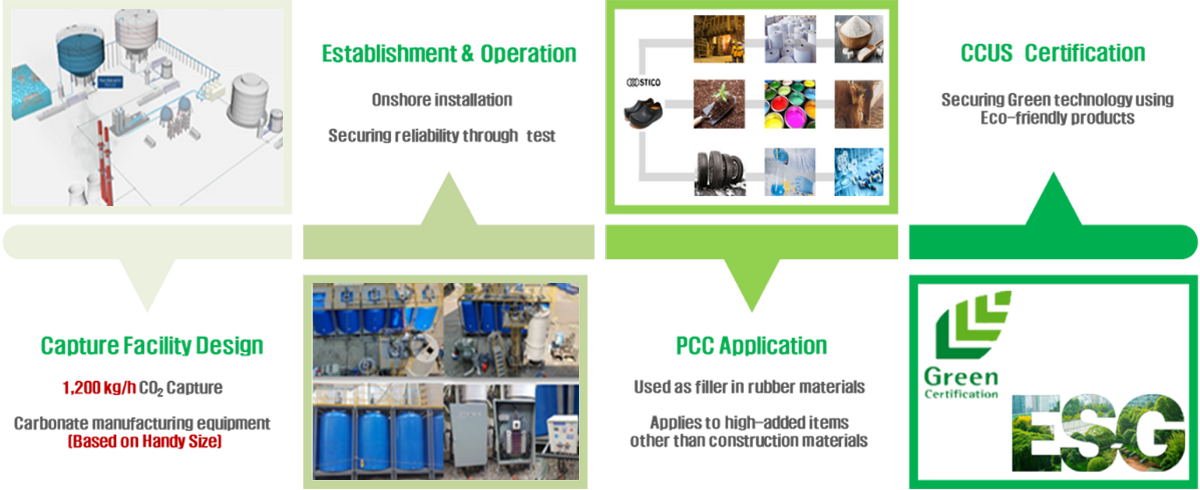
Utilization of the product
Depending on the CCUS technology applied, the product is either liquefied CO2 or carbonate.
CaCO3 (PCC) produced with our technology can be sold to the following market
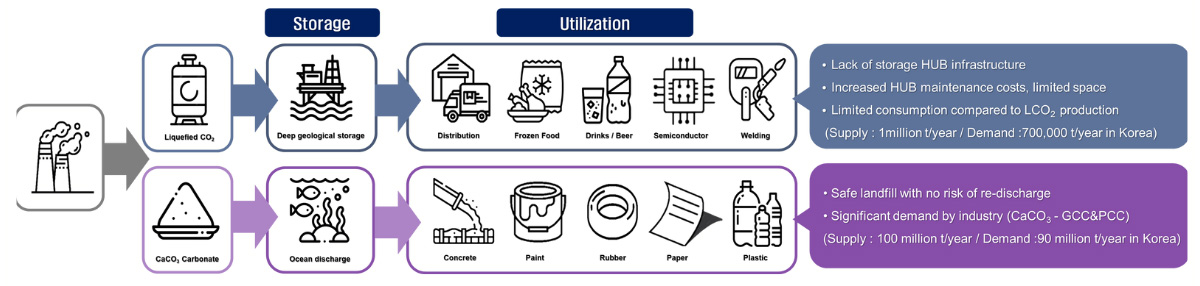
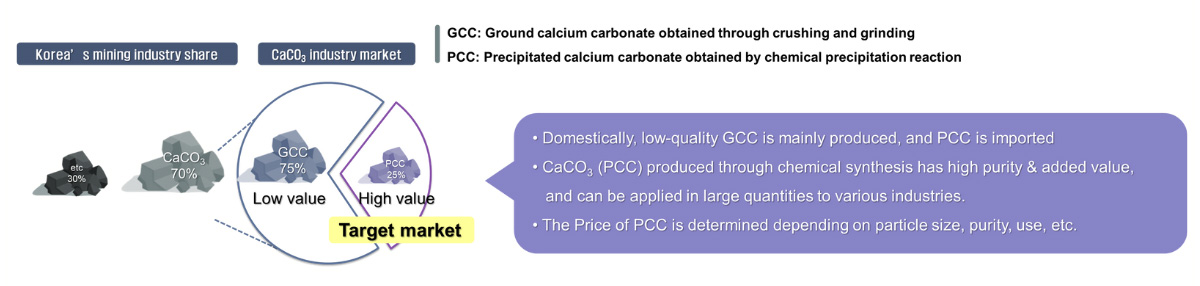

Purified CO2 can be used directly in carbonated water production, welding, dry ice, semiconductors, etc., but demand is very low compared to future supply.
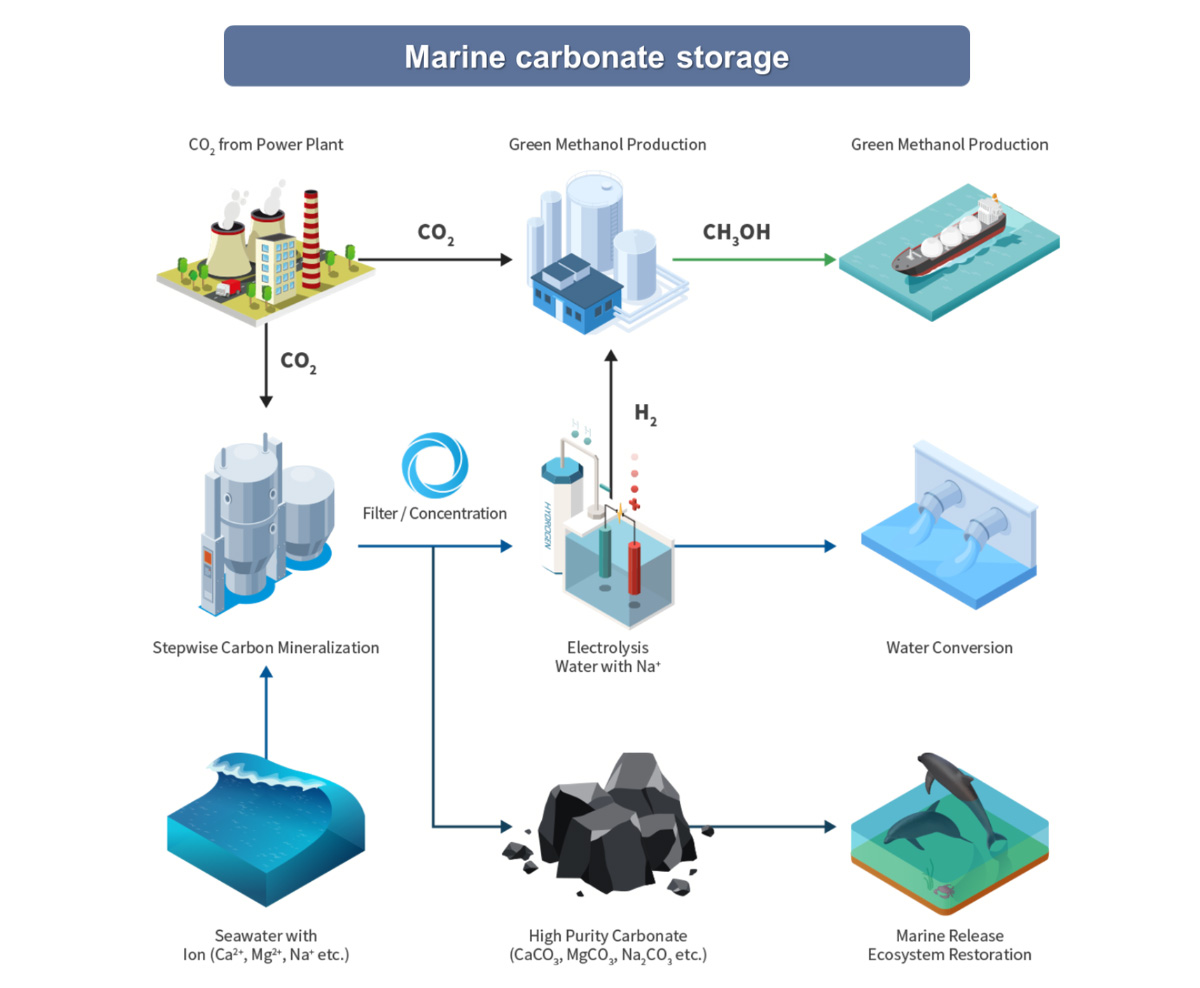
We increase the value of CCUS by applying technologies such as manufacturing and utilizing ceramic raw materials through carbonate mineralization, separating deep ocean water according to environmental impact assessment, and E-methanol synthesis.