Cations such as Ca, Mg, and Na exist in seawater, a semi-permanent resource, and produce carbonate, a stable mineral form, through reaction with CO2 under alkaline conditions. CO2 generated from power generation is captured and stored at a location where seawater is easily handled. This is different from the CCS process for producing liquefied carbonic acid. It does not require separate desorption, compression, or liquefaction processes and is easy to store. The reaction between alkaline salts and CO2 in seawater is a spontaneous exothermic reaction, and complex carbonates such as dolomite are created through ionic bonding between metals and non-metals.
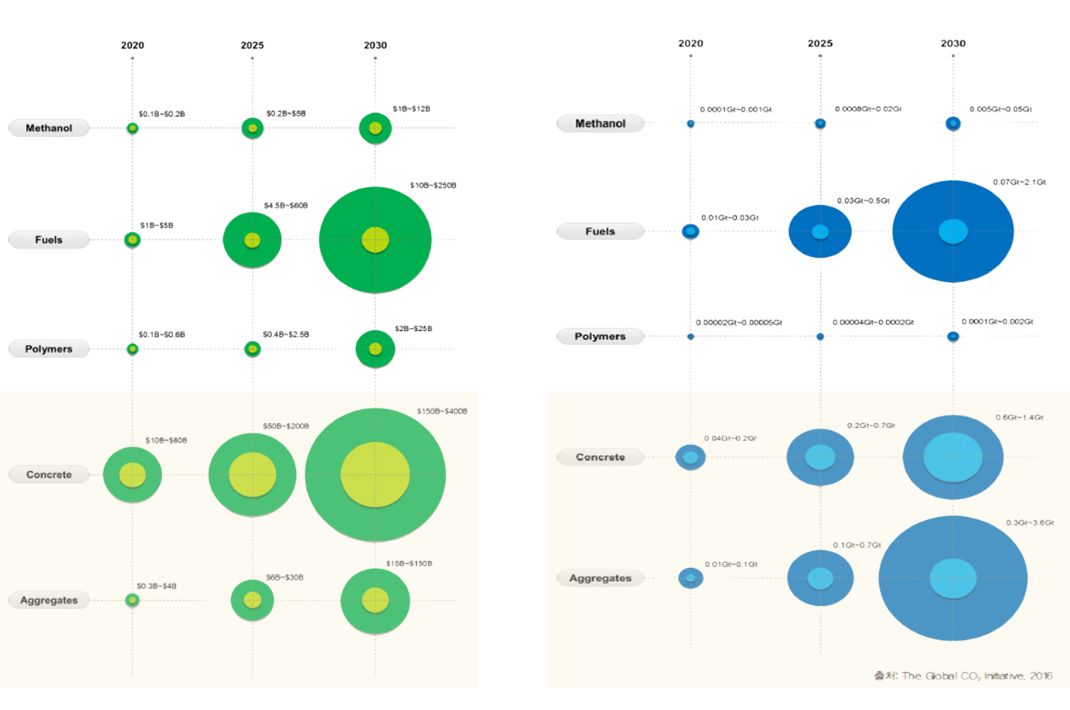
Carbon-using products such as fuel, chemical products, and building aggregates are expected to form a market of
up to $70 billion in 2020, and will grow at a CAGR of 13.8% in the future to form a market of up to $8,370 in
2030.
In the Intergovernmental Panel on Climate Change (IPCC) Special Report on Carbon Mineralization, CCUS technology
represents the highest potential storage capacity among carbon storage methods and is classified as a reliable
and operational technology. It is a permanent method and does not require long-term monitoring as there are no
CO2 leakage issues.
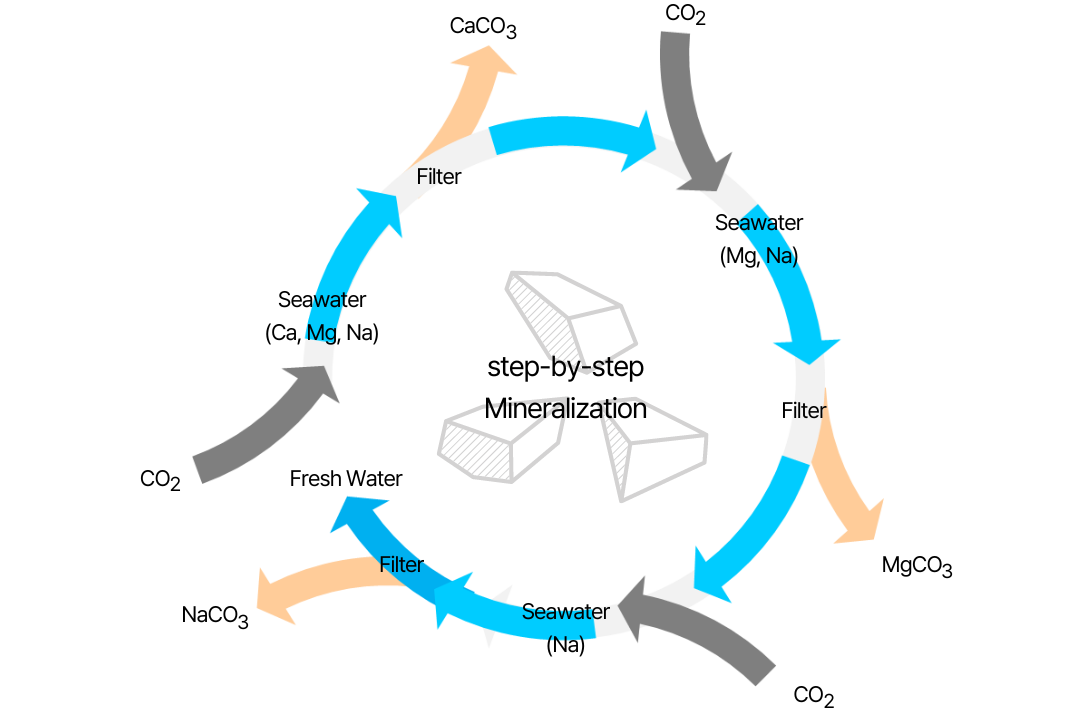
- MgCO3, CaCO3 : Paper, Plastics, Paint, Rubber, Heat insulating material, etc.
- Na2CO3 : Soap, Detergent, Glass materials, Chemical additives, etc.
The technology to individually produce carbonates using differences in solubility further increases the value of the product. The industrial field can be expanded by producing calcium, magnesium, and sodium carbonate in stages

Carbon dioxide storage carbonate manufacturing process using seawater

Thermal Power Plant Pilot Demonstration
[2017.05 ~ 2019.04]
- Control of solubility in carbonate
- Control of particle shape and size according to reaction conditions
- Standardization and stabilization of various carbonate production
- Study on properties of resin composition and applied products
Securing a step by step manufacturing process system
