More than a quarter of atmospheric CO2 is absorbed into the ocean. The phenomenon of lowering pH by
increasing
the hydrogen ion concentration in seawater is commonly called ‘ocean acidification’. The basicity of seawater
decreases, but it never becomes acidic below PH7. In other words, ‘ocean acidification’ refers to the process
of lowering the pH of seawater and its effects.
Since the Industrial Revolution, ocean acidity has increased by about 30%, and if atmospheric carbon dioxide
concentration continues to increase at the current rate, pH is estimated to decrease by about 0.2 to 0.4 by
the end of 2020. (The current pH of sea water is 8.2) The 21st century geological record shows that ocean
acidification has occurred several times in the past and was associated with the mass extinction of calcium
carbonate-based marine life about 55 million years ago. Although this occurred slowly over millions of years,
it took coral reefs more than a million years to recover.
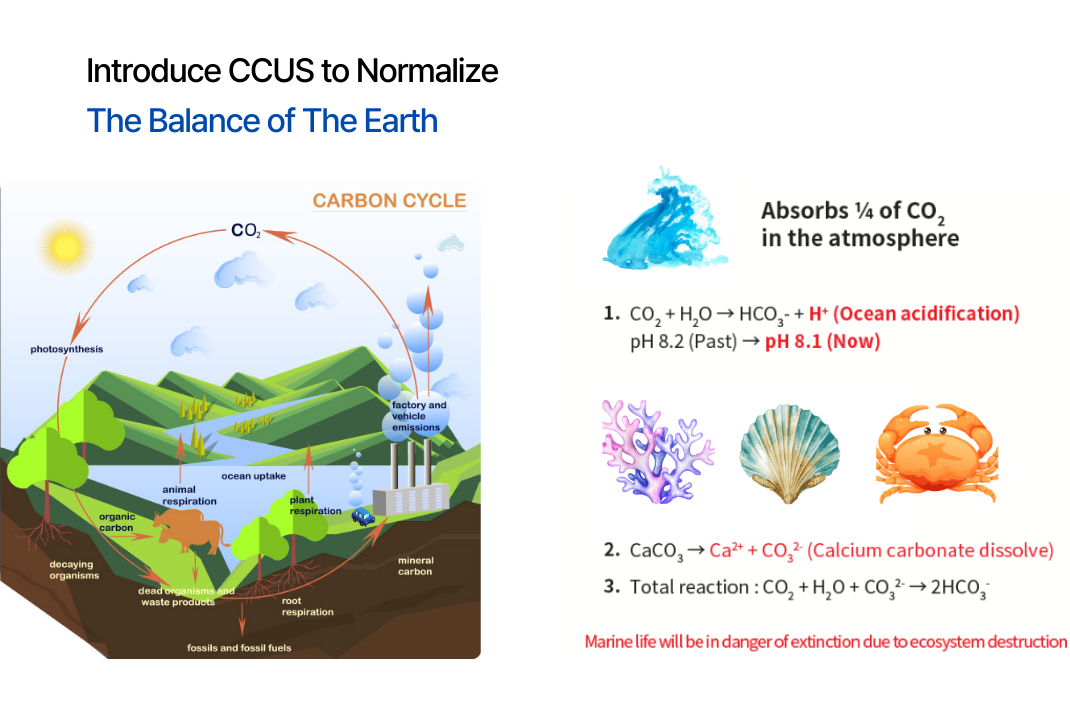
Meanwhile, current acidification is progressing more than 100 times faster than in the past, with pH lowering by about 0.1 over the past 250 years since the Industrial Revolution. If acidification continues at the current rate, corals will disappear from tropical waters within a few centuries, and marine life with calcium carbonate skeletons will also begin to dissolve in most polar waters. These changes will ultimately have serious impacts on food chains, biodiversity and fisheries resources.
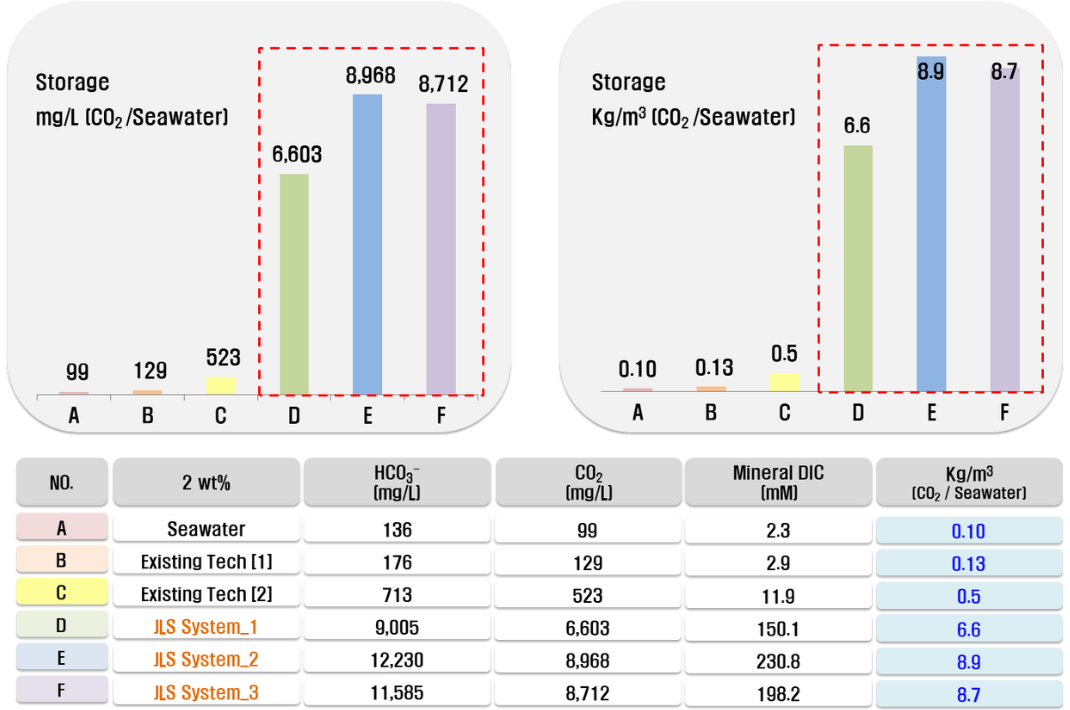
Carbonate is primarily created through a neutralization reaction between CO2 and alkaline salts in seawater, and as PH continues to decrease, carbonate is converted to bicarbonate and dissolved. As a result, the dissolved inorganic carbon concentration increases to 200mM, which is 150 times more concentrated than the 2mM inorganic carbon concentration of general seawater in natural conditions. The density of seawater with this inorganic carbonate ion concentration increases by 0.1 to 0.2%, and when compared to the density distribution by depth of seawater in the East Sea in Korea, it is equivalent to the density difference between the mixed layer and the water depth of 300M or less.
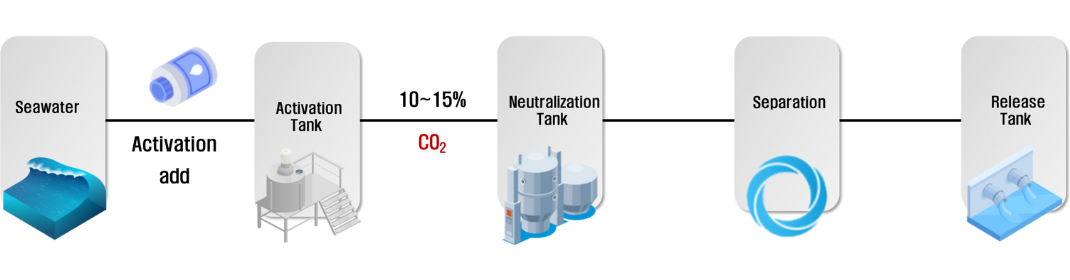
Seawater with excess carbonate ions dissolved in it has a higher density than the surface seawater and sinks. In order to safely isolate it in the deep seafloor, it is desirable to discharge it into a safe layer (section below the thermocline) with high pressure and low water temperature where there is no exchange of seawater between the upper and lower layers. The pH in this section is 7.4, which is different from the pH of surface seawater of 8.2. Seawater with a maximized concentration of bicarbonate ions is discharged at pH 7.4 and can be stored stably. This method can minimize the re-emission of high concentrations of dissolved inorganic carbon back into the atmosphere in the form of CO2. It can also reduce the impact on coastal environments where fishing and biological production are active.
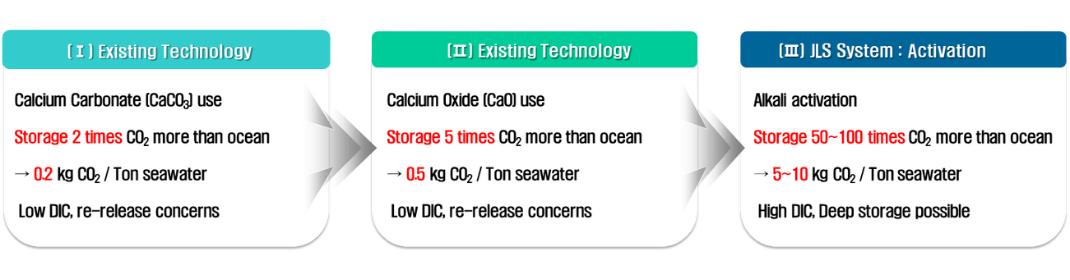
There are concerns about the impact on marine life in the discharge area and the acceleration of global warming when re-released, so environmental impact assessments must be continuously conducted. Currently, developments in carbon dioxide capture technology are continuously being made, but due to the need to store large amounts of CO2, EOR (Enhanced Oil Recovery) in oil and gas fields is biased towards CCS technology. Considering the limited isolation space such as saline water layers, JLS technology can be a groundbreaking carbon dioxide storage method.
