ECOSYSTEM RESTORATION
Seawater Alkalization
STICO Lab is advancing several carbon capture and storage opportunities around the world to help support our commitment to the Paris Agreement.

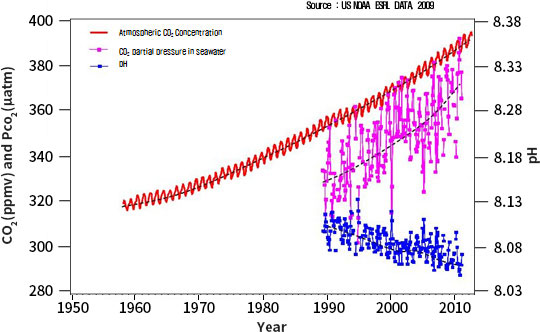
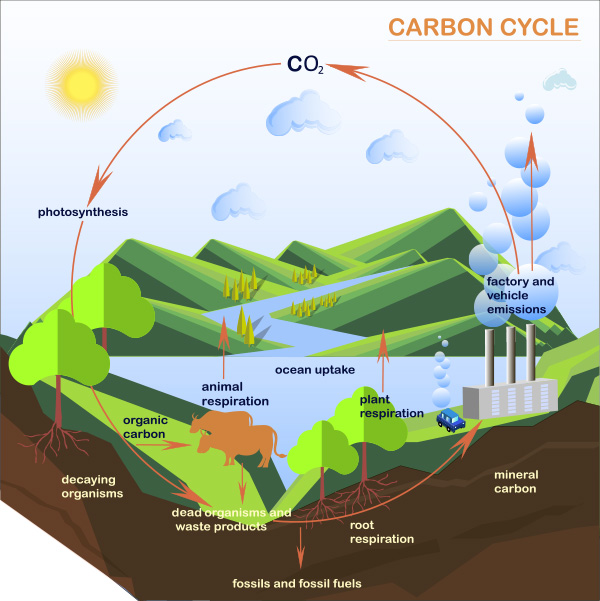
Since the Industrial Revolution, more than a quarter of the rapidly increasing atmospheric carbon dioxide
(CO2) is absorbed into the ocean.
The increase in the concentration of hydrogen ions in seawater to lower the pH is commonly referred to as
"Ocean acidification".
However, it should never be confused that the pH of the ocean will actually drop below 7 and become acidic.
This is because it is practically impossible for the pH of seawater to drop below 7. If ocean acidification
occurs, the basicity of seawater will decrease, but it will never change to acidity.
In other words, "ocean acidification" refers to the process of reducing the pH of seawater and its effects.

The acidity of the ocean has increased by about 30% since the Industrial Revolution, and if the atmospheric
carbon dioxide concentration con-tinues to increase with the current trend, it is estimated that the pH will
decrease by 0.2-0.4 by the end of the 21st century. According to the geological record, ocean acidification
has already occurred several times in the past, and acidification that occurred about 55 million years ago is
associated with the mass extinction of calcium carbonate-based marine species. Although this acidification has
progressed slowly over mil-lions of years, it has taken coral reefs over a million years to recover from it.
Meanwhile, the current acidification is progressing more than 100 times faster than the acidification of the
past, as the pH has been lowered by 0.1 for about 250 years since the Industrial Revolution. If acidification
proceeds at the current rate, corals will disappear from tropical waters within a few centuries and the
skeletons of marine organisms with calcium carbonate skeletons will begin to melt in most polar waters as
well. These changes will ultimately have a serious impact on the food chain, biodiversity and fisheries
resources.

The carbon mineralization process through the spontaneous reaction of carbon dioxide with an alkalized
solution through seawater electrol-ysis serves as a safe form of carbon dioxide storage.
In addition, when re-discharged to the sea, it becomes a supply medium for carbonate ions in seawater,
enabling restoration of the marine ecosystem.
